Dehydration Synthesis Of A Dipeptide
Chapter three. Amino Acids & Proteins
- 3.1 Biological Macromolecules
- 3.2 Types and Functions of Proteins
- 3.3 Amino Acids
- 3.4 Protein Structure
Introduction
We volition now begin our tour of the 4 major types of macromolecules establish in living organisms. The first blazon of molecule, proteins, are molecular machines that practise the piece of work of cells. They have a huge variety of structure and function. But before nosotros delve into how protein structure relates to protein office, we beginning have to discuss macromolecules.
3.1 | Biological Macromolecules
By the cease of this section, you volition exist able to:
- Name the 4 major classes of biological macromolecules.
- Understand the synthesis of macromolecules.
- Depict dehydration synthesis and hydrolysis reactions.
Biological macromolecules are large molecules, necessary for life, that are built from smaller organic molecules. There are four major classes of biological macromolecules: carbohydrates, lipids, proteins, and nucleic acids. Each is an of import cell component and performs a wide array of functions. Combined, these molecules brand upwards the majority of a jail cell's dry out mass (remember that water makes upwards the majority of its complete mass). Biological macromolecules are organic, meaning they contain carbon. In improver, they may incorporate hydrogen, oxygen, nitrogen, and additional minor elements.
3.1.1 Dehydration Synthesis Reactions
Well-nigh macromolecules are made from single subunits, or building blocks, called monomers. The monomers combine with each other using covalent bonds to class larger molecules known as polymers. In doing so, monomers release water molecules equally byproducts. This type of reaction is known as aridity synthesis (as well known as condensation), which means "to make while losing water."
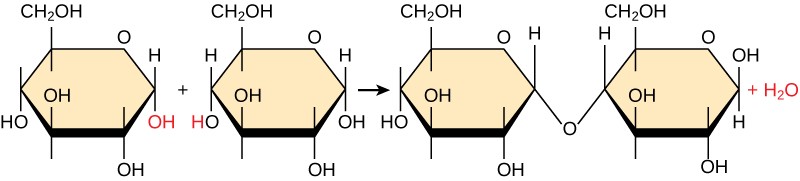
In a aridity synthesis reaction, the hydrogen of i monomer combines with the hydroxyl grouping of another monomer, releasing a molecule of water (Effigy 3.2). At the same time, the monomers share electrons and grade covalent bonds. As additional monomers join, this chain of repeating monomers forms a polymer. Different types of monomers can combine in many configurations, giving rise to a various grouping of macromolecules. Even one kind of monomer tin can combine in a multifariousness of means to form several unlike polymers: for example, glucose monomers are the constituents of starch, glycogen, and cellulose.
3.i.ii Hydrolysis Reactions
Polymers are broken down into monomers in a process known as hydrolysis, which means "to split h2o." (Figure 3.3). During these reactions, the polymer is broken into 2 components: i role gains a hydrogen atom (H+) and the other gains a hydroxyl molecule (OH–) from a split water molecule.
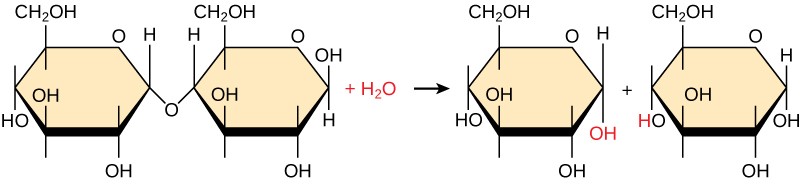
Aridity and hydrolysis reactions are catalyzed, or "sped up," past specific enzymes; dehydration reactions involve the germination of new bonds, requiring free energy, while hydrolysis reactions pause bonds and release energy. These reactions are similar for most macromolecules, but each monomer and polymer reaction is specific for its course. For case, in our bodies, food is hydrolyzed, or broken downwards, into smaller molecules by catalytic enzymes in the digestive system. This allows for piece of cake absorption of nutrients by cells in the intestine. Each macromolecule is cleaved downwardly past a specific enzyme. For example, carbohydrates are cleaved down past amylase, sucrase, lactase, or maltase. Proteins are broken down by the enzymes pepsin and peptidase, and by hydrochloric acid. Lipids are broken down by lipases. Breakup of these macromolecules provides energy for cellular activities.
iii.two | Types and Functions of Proteins
By the terminate of this section, you lot will exist able to:
- Describe the functions proteins perform in the cell and in tissues.
Proteins are one of the most abundant organic molecules in living systems and take the most various range of functions of all macromolecules. They are all, however, polymers of amino acids, bundled in a linear sequence. Proteins may be structural, regulatory, contractile, or protective; they may serve in transport, storage, or membranes; or they may be toxins or enzymes. Each cell in a living system may contain thousands of proteins, each with a unique function. Their structures, similar their functions, vary profoundly.
Structural proteins brand up the cytoskeleton within cells and the scaffold outside of cells. They include the keratin of our skin and the collagen of our connective tissue. Contractile proteins include actin and myosin, which allow muscles to contract. Antibodies that help mount an allowed response are proteins, as is hemoglobin, which transports oxygen in our blood. Jail cell membranes contain many proteins, including receptors, channels, and pumps, and many of the signaling molecules that demark to receptors, such as hormones, are proteins. Enzymes are proteins that catalyze biochemical reactions. The primary types and functions of proteins are listed in Table 3.1.
Table iii.1 Protein types and functions.
| T ype | Examples | Functions |
| Digestive Enzymes | Amylase, lipase, pepsin, trypsin | Help in digestion of nutrient by catabolizing nutrients into monomeric units |
| Ship | Hemoglobin, albumin | Carry substances in the blood or lymph throughout the body |
| Structural | Actin, tubulin, keratin | Construct different structures, similar the cytoskeleton |
| Hormones | Insulin, thyroxine | Coordinate the activity of different body systems |
| Defense force | Immunoglobulins | Protect the body from foreign pathogens |
| Contractile | Actin, myosin | Effect muscle contraction |
| Storage | Legume storage proteins, egg white (albumin) | Provide nourishment in early development of the embryo and the seedling |
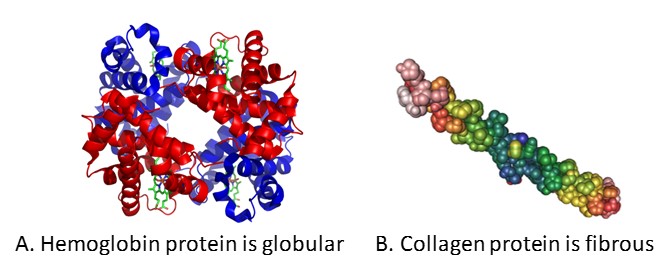
Reflecting their various functions, proteins take very diverse shapes and sizes. Some proteins, such every bit hemoglobin, are globular in shape whereas others, such equally collagen, are fibrous (Effigy 3.4). The shape of each poly peptide is critical to its function, and this shape is maintained by many unlike types of chemical bonds. Changes in temperature, pH, and exposure to chemicals may lead to permanent changes in the shape of the protein, leading to loss of function, known as denaturation.
3.iii | Amino Acids
By the end of this section, you will exist able to:
- Discuss the human relationship between amino acids and proteins.
- Describe the structure of an amino acid.
- Understand the peptide bond.
Amino acids are the monomers that make up proteins. All proteins are made up of different arrangements of the same 20 amino acids. Each amino acrid has the same fundamental structure, which consists of a central carbon cantlet bonded to an amino group (NH2), a carboxyl group (COOH), a hydrogen atom, and a variable "R" group (Effigy 3.5). The proper noun "amino acid" is derived from the presence of the amino group and the acidic carboxyl group.
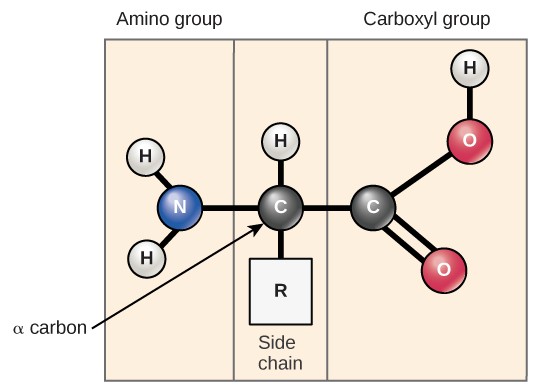
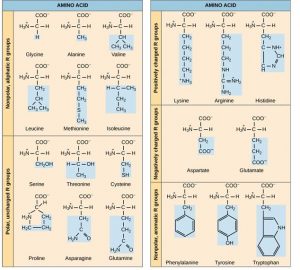
The same 20 common amino acids are nowadays in proteins from all species of life. Ten of these are considered essential amino acids in humans because the homo torso cannot produce them and they must be obtained from the diet. Each amino acrid differs merely in the R grouping (or side concatenation). The chemical nature of the R group determines the nature of the amino acrid (that is, whether it is acidic, basic, polar, or nonpolar). For instance, amino acids such as valine, methionine, and alanine are nonpolar or hydrophobic in nature, while amino acids such equally serine, threonine, and cysteine are polar and accept hydrophilic side chains. The side bondage of lysine and arginine are positively charged, while the side chains of aspartate and glutamate are negatively charged. (Effigy iii.6).
Amino acids are linked together into linear bondage called polypeptides. While the terms polypeptide and poly peptide are sometimes used interchangeably, a polypeptide is technically a polymer of amino acids, whereas the term protein is a polypeptide or polypeptides that accept a distinct shape and a unique role. The sequence and the number of amino acids decide the protein's shape, size, and function. After protein synthesis (translation), most proteins are modified. Only subsequently these modifications is the protein completely functional.
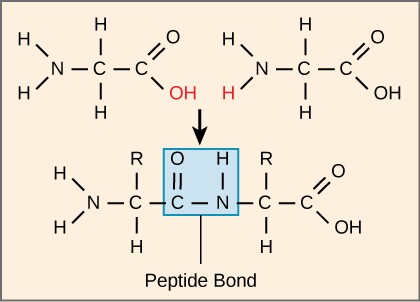
Each polypeptide has an N terminal, with a free amino group, and a C terminal, with a gratuitous carboxyl grouping. Amino acids are attached to other amino acids by covalent bonds, known every bit peptide bonds, which are formed past aridity synthesis reactions. The carboxyl group of ane amino acid and the amino group of the incoming amino acrid combine, releasing a molecule of h2o and forming a peptide bond (Effigy 3.7). Boosted amino acids are e'er added to the C terminus until the chain is consummate. The resulting polypeptide concatenation has a peptide backbone (or carbon-nitrogen backbone), that is identical for all proteins, with the variable R groups extending off of the backbone. Polypeptides differ from each other only in the order of the R groups.

The Evolutionary Significance of Cytochrome cCytochrome c is a protein that is important for cellular respiration. Sequencing the cytochrome c poly peptide from different species has shown that at that place is a considerable corporeality of amino acid sequence homology amid dissimilar species. Evolutionary kinship tin be assessed by measuring the similarities or differences amidst various species' DNA or protein sequences.Human cytochrome c contains 104 amino acids. 37 of these amino acids appear in the same position in samples of cytochrome c from a variety of unlike species. Human and chimpanzee cytochrome c is identical and human and rhesus monkey cytochrome c differ in only ane amino acid. Surprisingly, human and yeast cytochrome c also differ in just one amino acid.
3.4 | Poly peptide Structure
Past the cease of this department, you lot volition exist able to:
- Explain the four levels of protein organization.
- Describe the ways in which protein structure and function are linked.
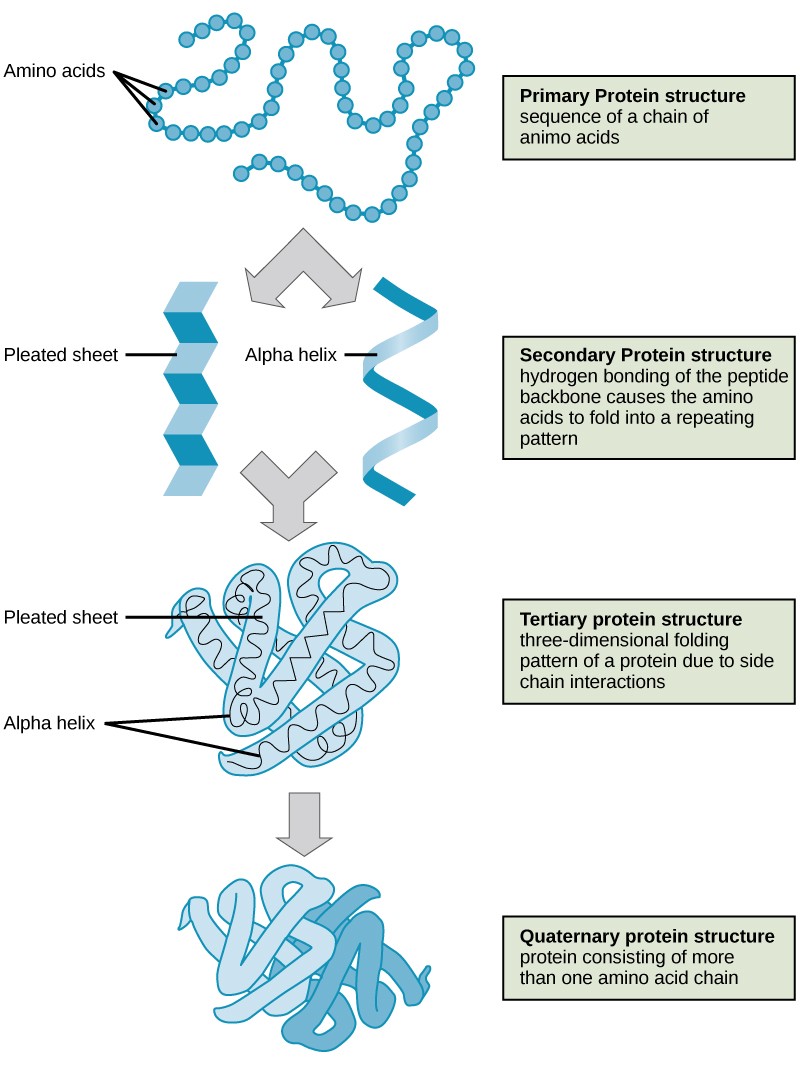
The structure of a protein is critical to its function. For case, an enzyme can demark to a specific substrate at a site known as the active site. If this agile site is altered because of local changes or changes in overall protein structure, the enzyme may be unable to demark to the substrate. To understand how the protein gets its final shape or conformation, we demand to understand the four levels of protein construction: primary, secondary, tertiary, and quaternary.
3.4.one Primary Structure
The unique sequence of amino acids in a polypeptide chain is its chief structure. For example, the pancreatic hormone insulin has two polypeptide chains, A and B, which are linked together by disulfide bonds. The primary construction of each chain is indicated by three-letter abbreviations that represent the names and gild of the amino acids. The Northward final amino acid of the A chain is glycine, whereas the C terminal amino acid is asparagine (Effigy iii.eight). The sequences of amino acids in the A and B chains are unique to insulin.
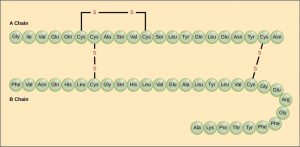
The amino acrid cysteine (cys) has a sulfhydryl (SH) group as a side chain. 2 sulfhydryl groups can react in the presence of oxygen to course a disulfide (S-South) bond. Two disulfide bonds connect the A and B chains together, and a third helps the A concatenation fold into the correct shape.
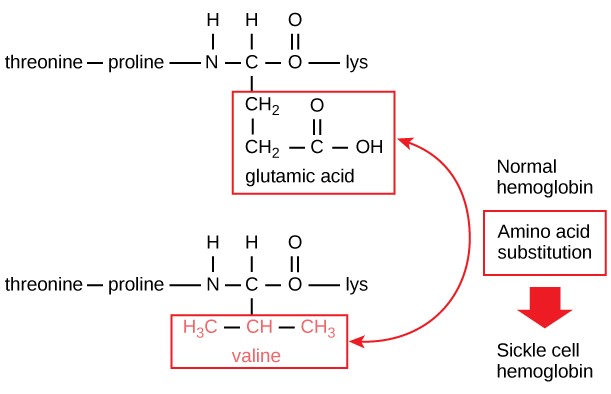
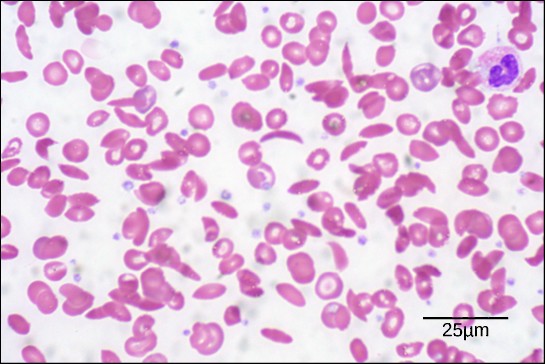
The unique primary sequence for every poly peptide is adamant by the cistron that encodes the protein. Fifty-fifty a modest change in a gene tin can lead to a different amino acid being added to the growing polypeptide chain. For case, in the human genetic disease sickle cell anemia, the hemoglobin β chain (a small portion of which is shown in Figure 3.9A) has a single amino acrid substitution (valine for glutamic acrid). This change of 1 amino acid in the chain causes hemoglobin molecules to course long fibers that distort red claret cells into a crescent or "sickle" shape, which clogs arteries and leads to serious health problems such as breathlessness, dizziness, headaches, and intestinal hurting (Figure 3.9B).
3.4.2 Secondary Construction
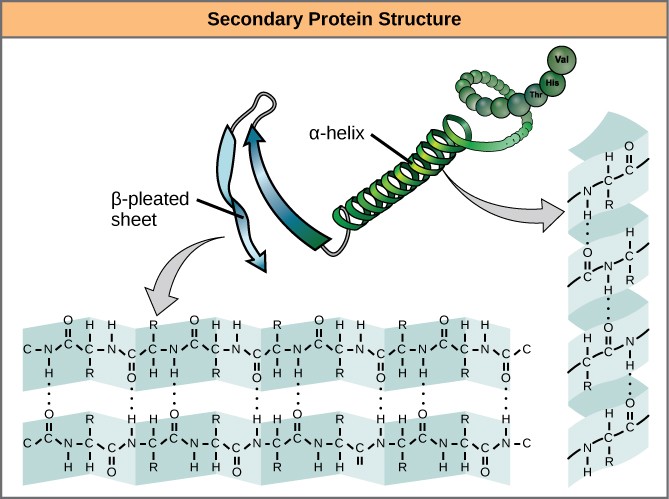
The local folding of the polypeptide in some regions gives rise to the secondary structure of the protein. The most common are the α -helix and β -pleated sheet structures (Figure iii.10). Both structures are formed by hydrogen bonds forming between parts of the peptide backbone of the polypeptide. Specifically, the oxygen atom in the carbonyl group in i amino acrid interacts with some other amino acrid that is four amino acids farther forth the concatenation.
3.four.3 Tertiary Structure
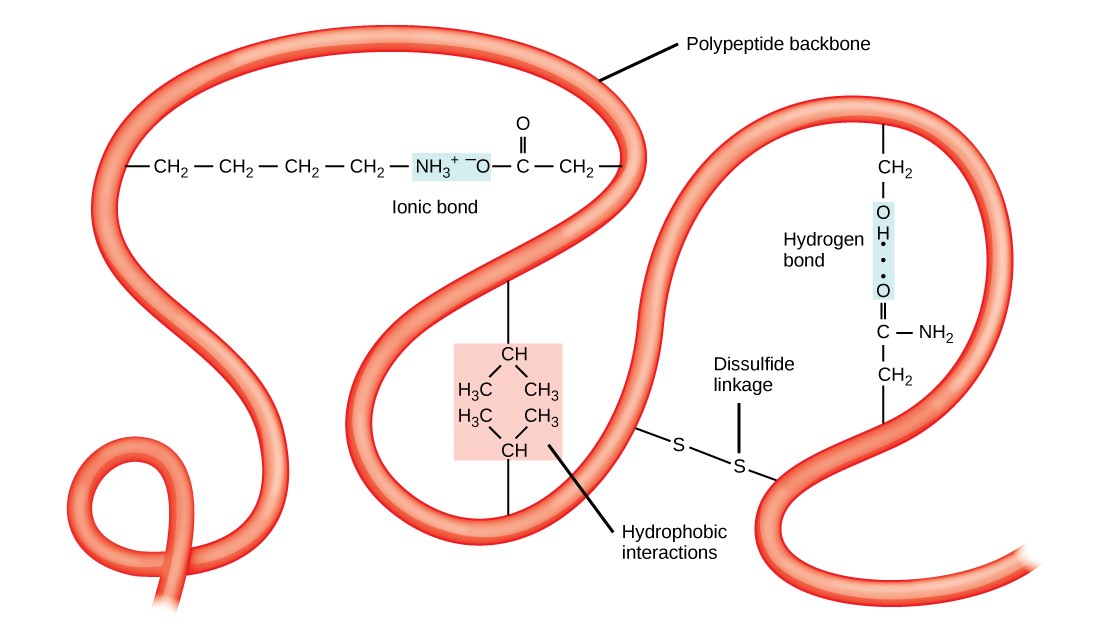
The unique three-dimensional structure of a polypeptide is its third construction (Figure three.xi). This construction is primarily due to interactions amid R groups. For example, R groups with like charges are repelled past each other and those with unlike charges are attracted to each other via ionic bonds. When protein folding takes place in a watery environment, such every bit that found inside cells, the hydrophobic R groups of nonpolar amino acids lay in the interior of the poly peptide, while the hydrophilic R groups confront out. Hydrophobic R groups also collaborate with each other through van der Waals forces.Interaction betwixt cysteine side bondage forms disulfide linkages, which are the only covalent bond formed during protein folding. All of these interactions determine the concluding iii-dimensional shape of the protein. When a protein loses its iii-dimensional shape, it may no longer be functional.
iii.4.iv Quaternary Structure
In nature, some proteins are formed from several separate polypeptides, known as subunits. The interaction of these subunits forms the 4th structure of a poly peptide. Weak interactions between the subunits help to stabilize the overall structure. For case, silk is a fibrous poly peptide that results from hydrogen bonding between different chains.
The four levels of poly peptide structure (primary, secondary, tertiary, and quaternary) are illustrated in Figure three.i.
three.4.5 Denaturation and Protein Folding
If a protein is subject field to changes in temperature, pH, or exposure to chemicals, information technology may lose its shape, a process called denaturation. The chief structure of the poly peptide is not changed by denaturation merely some or all of the folding is lost. Denaturation is often reversible, allowing the protein to resume its function. Sometimes denaturation is irreversible, leading to permanent loss of role. One example of irreversible protein denaturation is cooking an egg white. Unlike proteins denature under unlike conditions. For case, bacteria from hot springs have proteins that function at temperatures close to humid. Although stomach acid denatures proteins as part of digestion, the digestive enzymes of the stomach retain their activity under these conditions.
Correct folding of proteins is critical to their function. Although some proteins fold automatically, recent research has discovered that some proteins receive assist in folding from poly peptide helpers known as chaperones.
Dehydration Synthesis Of A Dipeptide,
Source: https://rwu.pressbooks.pub/bio103/chapter/amino-acids-and-proteins/
Posted by: millermrsawas.blogspot.com


0 Response to "Dehydration Synthesis Of A Dipeptide"
Post a Comment